Agreement
We use cookies on our website to provide you with the best possible experience. By clicking "Accept All" you agree to the use of all cookies and to our privacy policy.
The digital signature in medical technology
Sign 100% legally valid & FDA-compliant
100% GDPR compliant web solution
Measurable acceleration of all release processes
Highest e-signature standard - Made in Europe
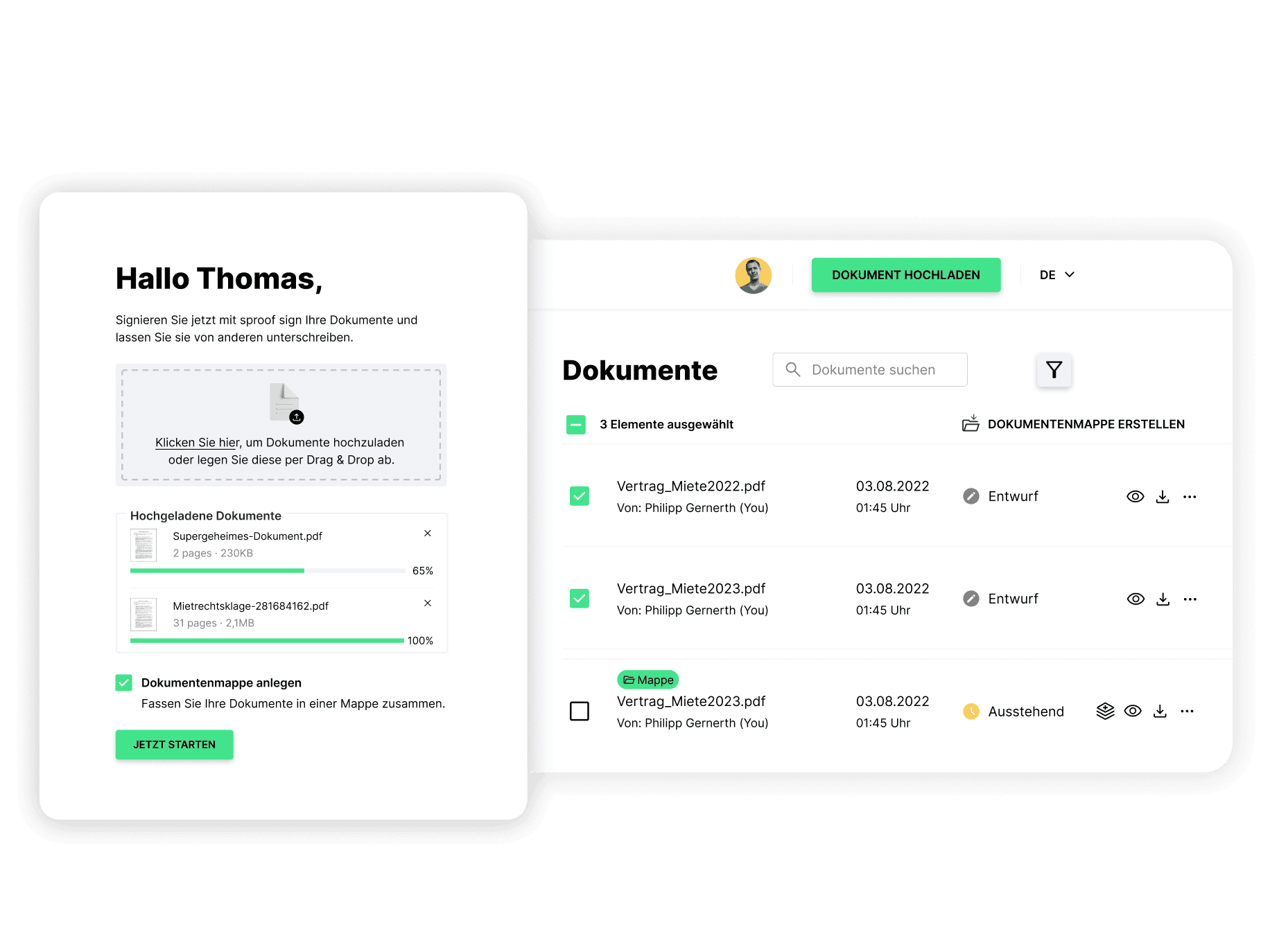
Ready to go, no setup or installation required

Maximum speed and efficiency
Integrated into any IT infrastructure in a flash, all approval and documentation processes, from production to sales, are measurably accelerated.

Maximum safety & conformity
sproof sign fully complies with all industry guidelines for electronic signing including FDA (Part 11), eIDAS and ISO 27001 (server hosting).

Highest possible data protection
All services are hosted within the EU and without the involvement of third countries (no US involvement). The highest level of data protection is not an add-on for us.



Top companies with the highest standards of security and compliance are already signing with Europe's all-in-one platform for digital signing.
The all-in-one e-signature.Made in Europe for Europe.
The all-in-one e-signature.Made in Europe for Europe.
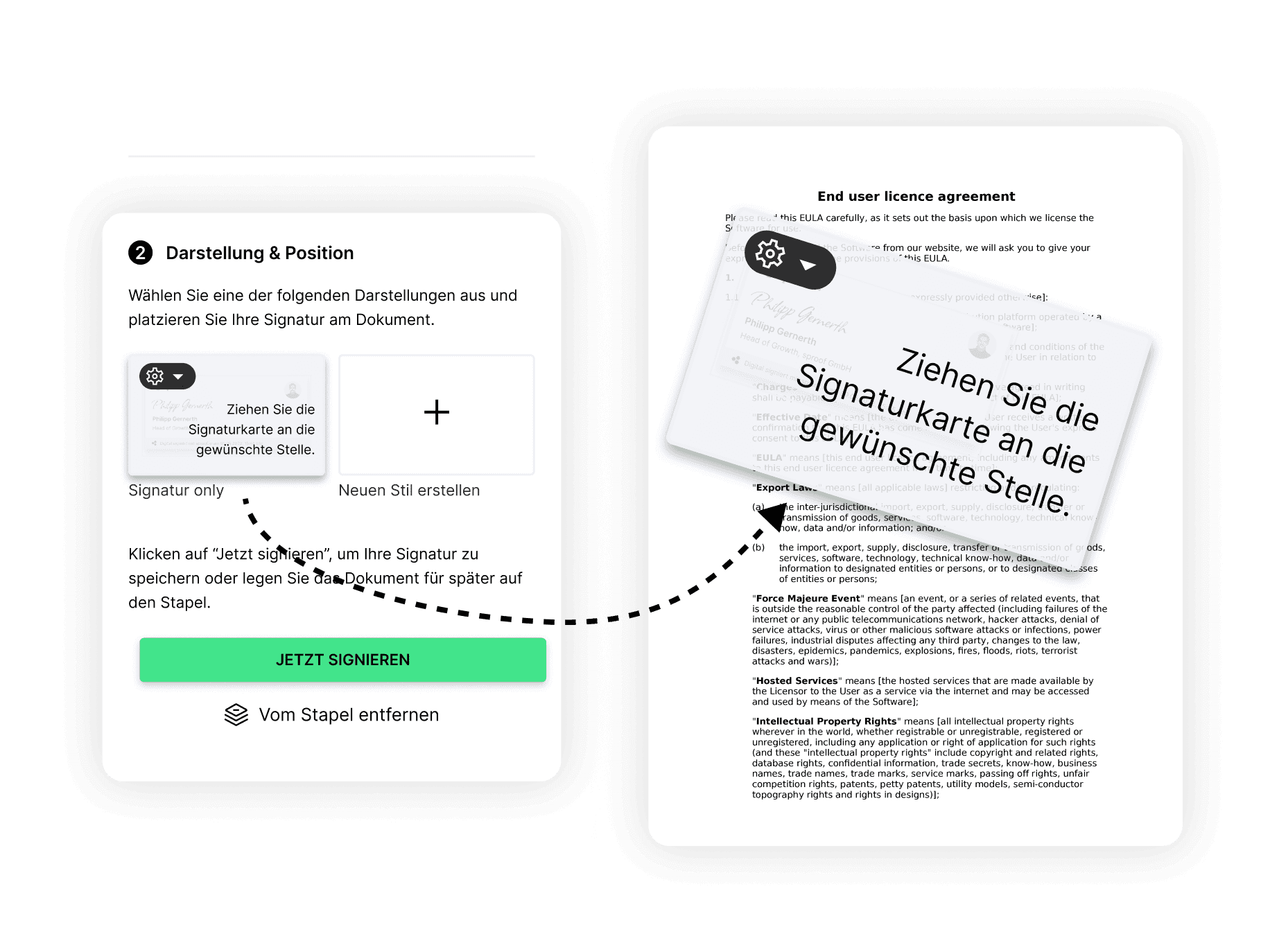
eSignatures (AES & QES) throughout Europe online.
All important contracts secured & managed in one place
Own company stamp & certificate.
Why does medical technology benefit from the digitized signature process?
Medical technology is subject to very strict regulations and standards (MDR, FDA, etc.). There is a good reason for this: the highest quality standards are a prerequisite for the health and safety of patients.
All processes relating to the design, manufacture and delivery of finished medical devices must be fully documented by law. This results in an unmanageable amount of bureaucracy. Almost all processes involved require approvals: The signature is an essential sub-step or often the critical bottleneck in many processes.
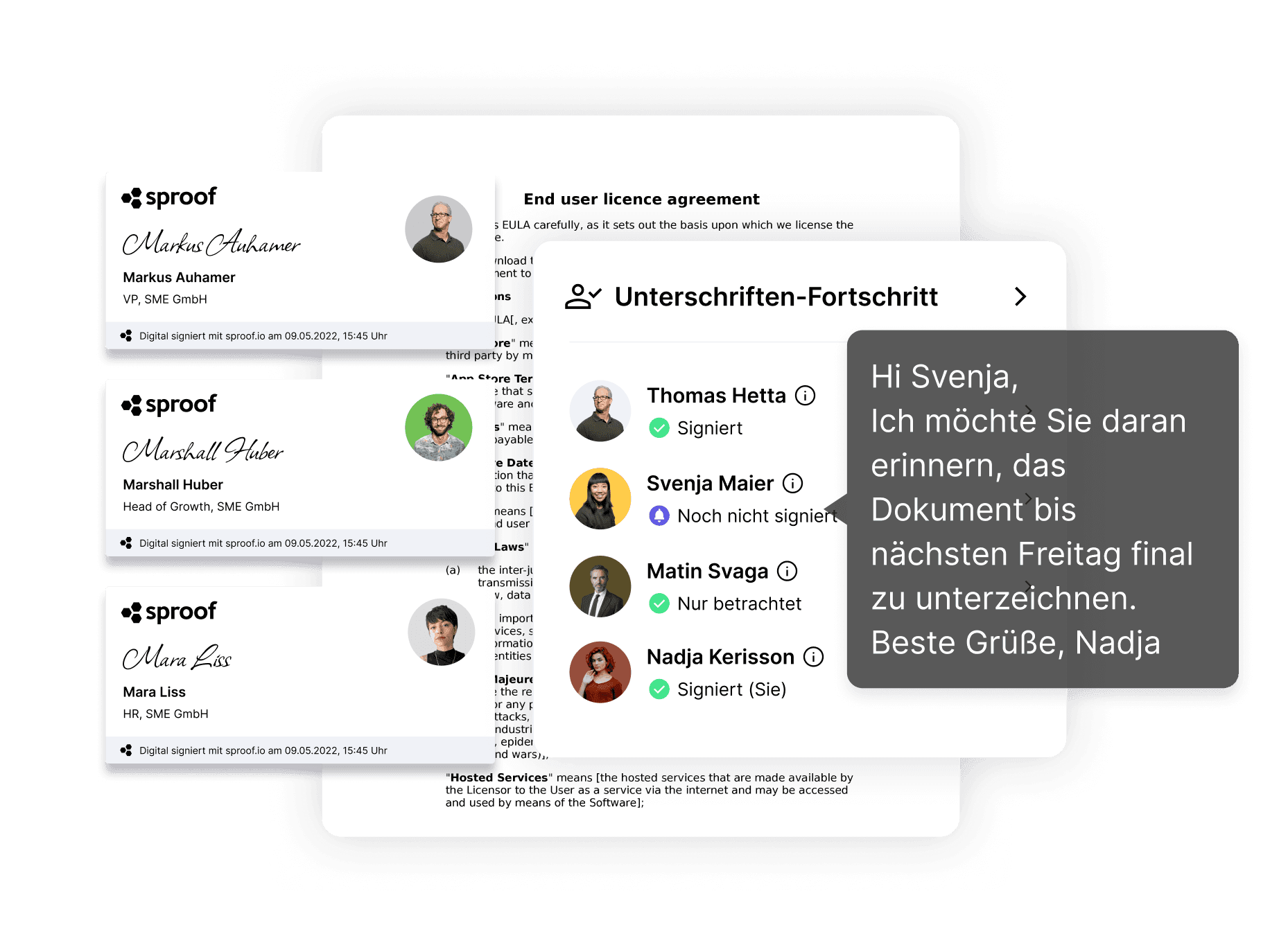
Speed up your company with the digital signature!
A digital signature workflow helps
- increase process speed 2) ensure security & conformity thanks to maximum probative value 3) improve usability within the company and in collaboration with external parties.
We at sproof sign have committed ourselves to precisely these high standards and offer a solution that makes it possible to significantly support all decisive use cases in the medical technology industry.

W&H is a leading global manufacturer and developer of medical technology products, specializing in the dental industry.With the introduction of sproof sign, the internal product development cycle has been greatly accelerated and streamlined.
W&H
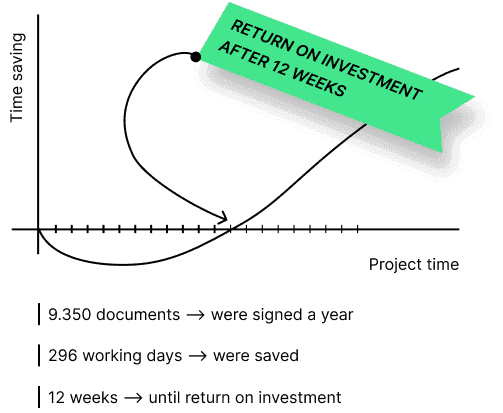

"After 12 weeks, the digital signature has paid off for us."
Ingrid Putzhammer, Vice President Global HR, Sony DADC
Digitization can pay off. We can calculate the added value of our solution for your application. For our customer Sony DADC, the introduction of sproof sign paid for itself after just 12 weeks.
Excellent e-signature platform. For excellent customers.


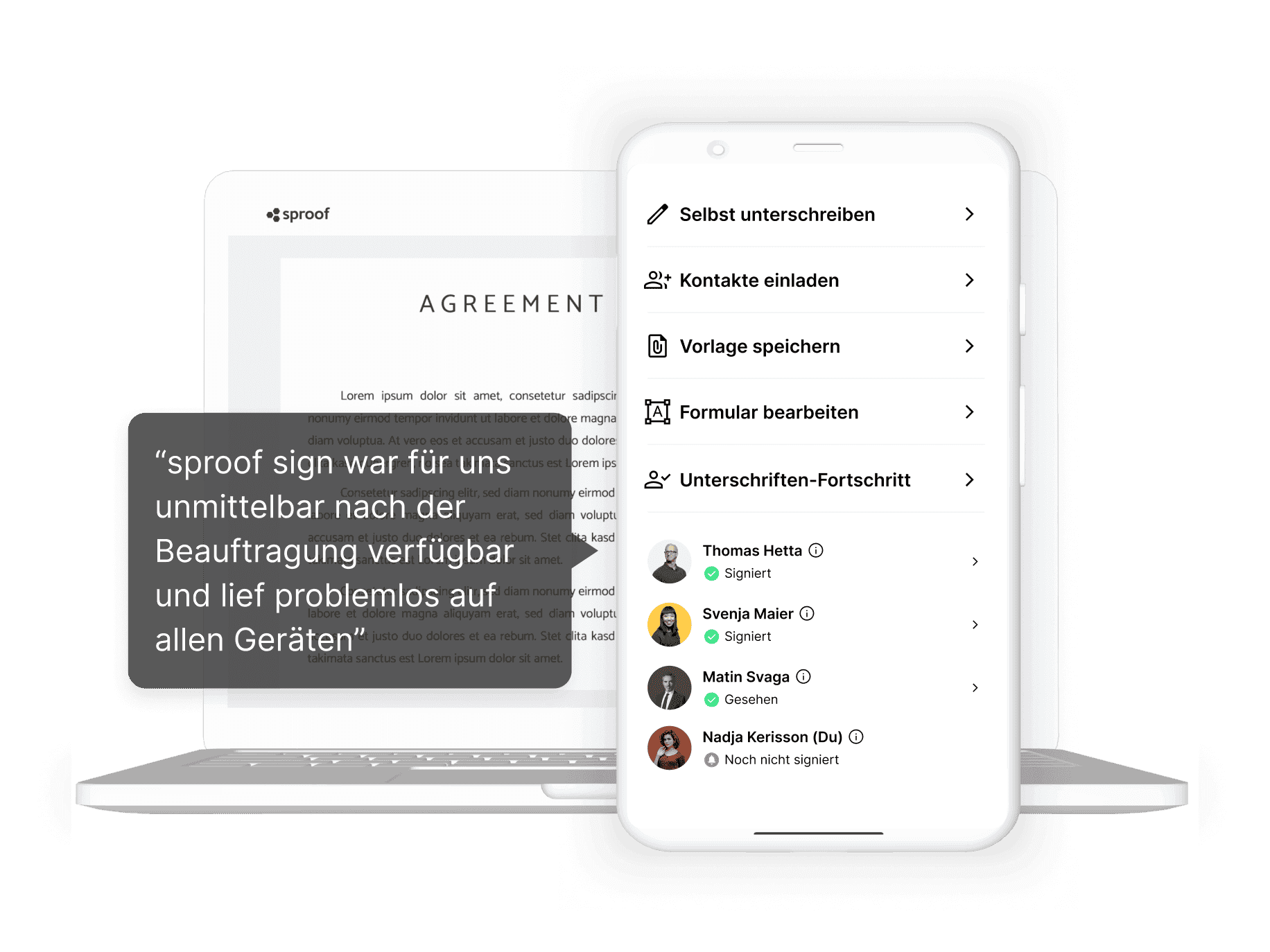
sproof sign is a 100% GDPR web application. The cloud can be integrated into your IT landscape in no time at all. We are known for our high flexibility and the best support.

Control and schedule your signature processes and manage your team independently. The simple API enables easy integration into the existing IT infrastructure.

sproof sign is integrated into the most important tools and thus enables you to have lean signature workflows and approval processes.
Frequently asked questions
Can I test sproof sign with my company for free?
Why should companies rely on European tools?
Which legal, security and compliance regulations does sproof sign meet?
Which e-signature standards does sproof sign offer for your company?
When do you need a qualified electronic signature (QES)?
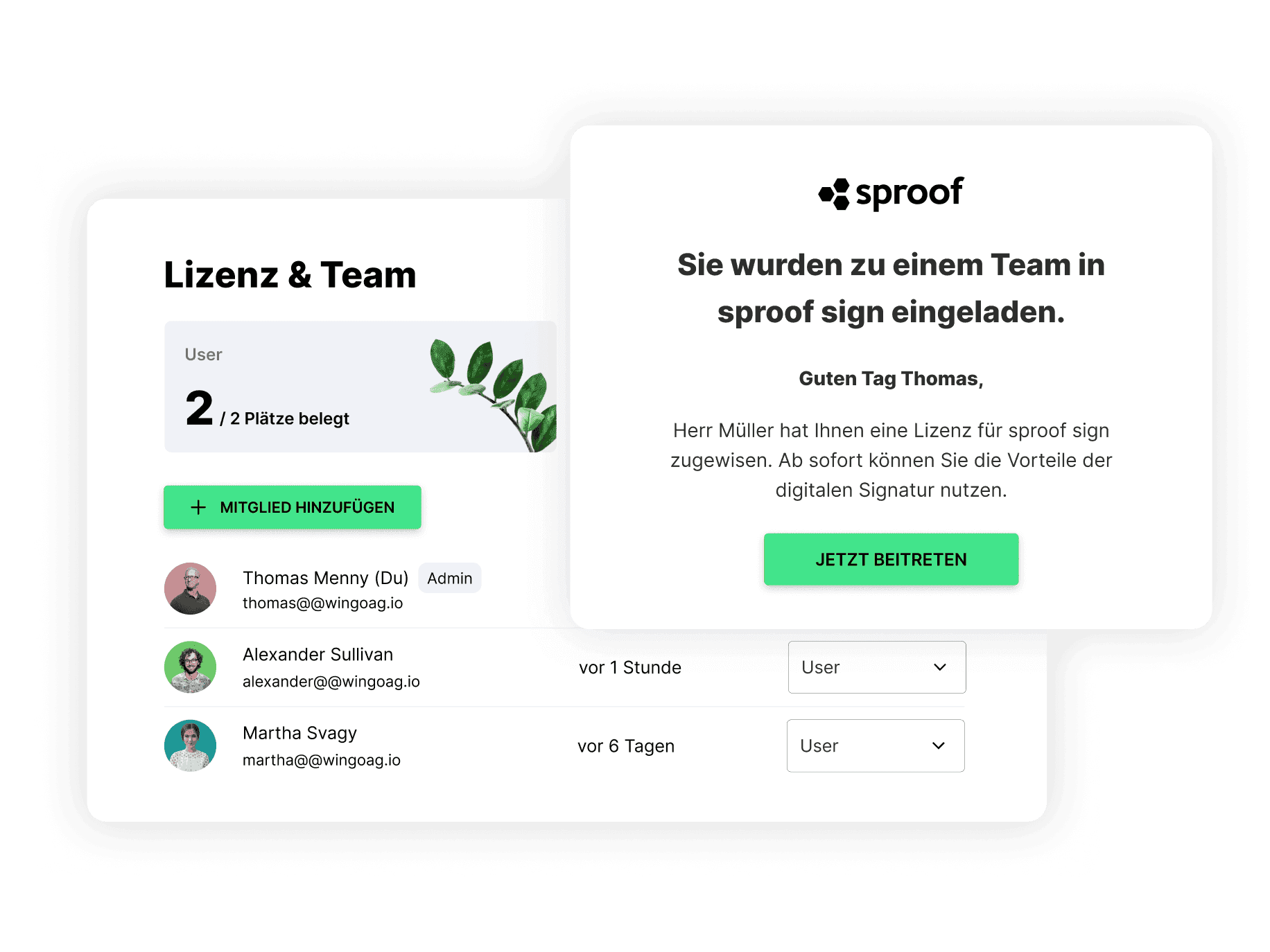
How does getting started with sproof sign work from single user to large enterprise?
Can a team & signature quota be managed independently with sproof sign?
How do I get a digital signature?
Can a company branding incl. company stamp and certificate be integrated in sproof sign?